New paper in Vaccine shows staggered age targeting and the durability of protection will be key to strategic and efficient vaccination in South Africa.
When an HIV vaccine is ready to launch in South Africa, an efficient implementation strategy needs to be ready. I worked with 3 women scientists in South Africa, 3 academic men in the UK, and 3 brilliant mathematicians in Seattle to help inform the strategy. We published our results in the journal Vaccine. You can read the open-access paper here.
Our study, funded by Bill and Melinda Gates, highlights the need to exploit different vaccination targeting and roll-out scenarios to maximize the population-level impact of a partially effective HIV vaccine in South Africa. Our model suggests that vaccinating population cohorts aligned with the ages of highest HIV incidence (i.e. 18-year-old women and 23-year-old men) and including continued boosting for up to 10 years could avoid about 13% of new HIV infections in the coming decades.
How long will the protection last?
Durability is how long a vaccine will continue to protect someone from infection. This is the math we used to describe it, based on what was learned from a similar vaccine candidate that was tested in humans:


Right now, the Pox-Protein Public-Private Partnership (P5) is supporting a large phase 2b/3 trial called HVTN 702 [1, 2]. It began enrolling South African patients in 2016 to test the preventative efficacy of an HIV vaccine in humans. We’ll know more about the efficacy and durability of HIV vaccine protection when the trial results are announced.
A vaccine with modest efficacy, vaccine efficacy at least 50%, could have substantial public health impact and significantly decrease the incidence of new infections in heavily burdened areas of the world.
Russel and Marovich, expert members of the P5 partnership
What will be most efficient?
The objective of our modeling study was to forecast HIV infections, disability-adjusted life-years (DALYs), and healthcare costs from a South African government payer perspective over a 30-year time horizon, from year 2018 to 2047. We wanted to compare a reference case for a world with no HIV vaccine to different implementation strategies for initiation of HIV vaccination.
A stochastic individual-based network model
Christian Selinger led the development of a detailed stochastic individual-based network model of disease transmission calibrated to the HIV epidemic using the Epidemiological MODeling software (EMOD) at the Institute for Disease Modeling (IDM). Here is the EMOD source code on GitHub.
Using an individual-based model, it was possible to apply a time-varying course of vaccine efficacy to each individual according to his or her own timing of vaccination and adherence to the booster series. This makes the model well suited for a nuanced analysis of the anticipated time-dependent efficacy of the pox-protein HIV vaccine regimen.

What we learned
Our analysis showed that this partially effective HIV vaccine could prevent, at catch-up vaccination with 60% coverage, up to 941,000 (15.6%) new infections between 2027 and 2047 assuming current trends of antiretroviral treatment. An impact of up to 697,000 (11.5%) infections prevented could be achieved by targeting age cohorts of highest incidence.
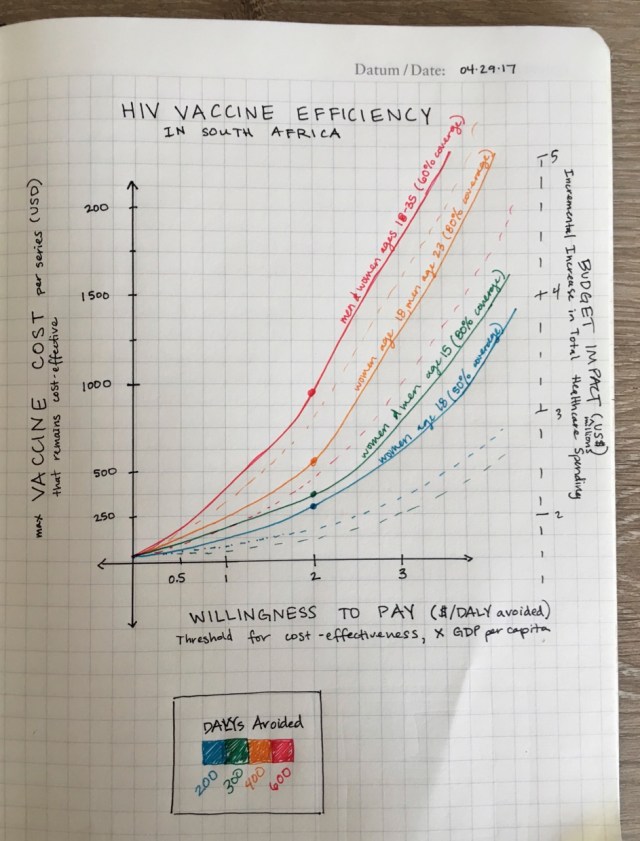
We learned that it is better to target women that are, on average, 5 years younger than the men targeted because of the sexual network dynamics. Considering the vaccine durability, an optimal roll-out strategy in South Africa could focus on 18-year-old women and 23-year-old men.
To vaccinate 80% of the population over 20 years will require 8 million vaccine series regimens. Catch-up vaccination would require a lot more product in the early years, with reduced demand in later years, totaling about 15 million regimens over 20 years.
Economic evaluation
If antiretroviral treatment scale-up is achieved, vaccination could be cost-effective at a total cost of less than $385 and $62 per 10-year series (cost-effectiveness thresholds of $5,691 and $750). Among the simulated vaccination strategies, catch-up vaccination for ages 18–35 is the most efficient when compared to cohort vaccination, since ART cost savings as well as averted DALYs are high, despite very similar maximum vaccine cost.

Key takeaway
The roll-out of a partially effective, rapidly waning vaccine alone will not eliminate HIV as a public health priority in South Africa. Vaccination will need to be performed in parallel with continued innovation in HIV prevention technologies.
Our research team in three corners of the world
Dr. Christian Selinger, previously at the Institute for Disease Modeling and currently at the Universite de Montpellier, led the modeling effort and did most of the work.
I was honored by the opportunity to work on this modeling study with several heroes I admire. (Learn more about Glenda Gray, a badass and my hero). Our research team was spread out in three corners of the world.
- Seattle: Christian Selinger, Anna Bershteyn, Dobromir Dimitrov, and me
- South Africa: Linda-Gail Bekker, Helen Rees, and Glenda Gray
- UK: Paul Revill, Tim Hallett, and Andrew Phillips

For those of you not familiar, the Institute for Disease Modeling prepares research for presentation to decision makers including Bill and Melinda Gates, GAVI, World Health Organization, and others for real time influence on global health policy. The Institute is now fortunate to have the leadership of an experienced infectious diseases health economist, Dr. Marita Zimmermann. Zimmermann enriches mathematical models of malaria, TB, and other infectious diseases with costs and benefits to assess the value of interventions.
Acknowledgements
This work was supported by Bill and Melinda Gates through the Global Good Fund.
We would also like thank the South African government, academic leaders, and community stakeholders who participated in one-on-one interviews to ensure that our analysis reflected the realities of the HIV epidemic and the health system in South Africa.
References
- Russell ND, Marovich MA. Pox-protein public private partnership program and upcoming HIV vaccine efficacy trials. Curr Opin HIV AIDS 2016;11(6):614–9. https://doi.org/10.1097/COH.0000000000000322.
- Pivotal Phase 2b/3 ALVAC/Bivalent gp120/MF59 HIV Vaccine Prevention Safety and Efficacy Study in South Africa. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/ NCT02968849.
- Selinger C, Bershteyn A, Dimitrov D, Adamson B, Revill P, Hallett T, Phillips A, Bekker LG, Rees H, Gray G. Targeting and Vaccine Durability Are Key for Population-level Impact and the Cost-Effectiveness of a Pox-Protein HIV Vaccine Regimen in South Africa. Vaccine. Volume 37, Issue 16, 10 April 2019, Pages 2258-2267. https://doi.org/10.1016/j.vaccine.2019.02.073
